here is a great deal of confusion about testosterone replacement therapy and whether or not people should use it. On the one hand, companies promote it to consumers as if it will solve many of their problems, but on the other, you’ll often see ads from attorneys for people who want to sue certain TRT manufacturers. After reading Part 1 of this series, you’ll know more about the basics of testosterone replacement therapy (TRT), including dosage, administration, bioidentical hormones, muscle growth, aromatization side effects, and inhibitors. Part 2 shares the potential risks associated with TRT.
Part 2: Risks
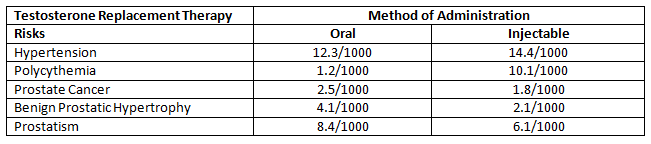
TRT has been associated with a number of risks, including increased acne, water retention, more frequent urination, increase in breast tissue, and minor shrinkage of the testicles[1]. Some of the more serious risks of TRT include potential development of prostate cancer, liver toxicity, hyperviscosity (thick blood), erythrocytosis (increase in the total red cells), the worsening of untreated sleep apnea, and severe heart failure[2]. A 2013 study identified the risks of TRT for a number of conditions per 1000 people[3].
This table helps to put the risk into perspective. I’ll discuss more about this later in the article.
TRT and Risk of Cardiac Events and Stroke
At the beginning of this article, I mentioned how lawyers are offering to help people who have been prescribed TRT in cases of heart attack or stroke. The relationship between TRT and adverse heart-related outcomes has received the most attention. Concern about this risk was raised by a number of recent studies.
A 2010 study investigated the adverse effects of using transdermal testosterone gel among 209 65-year-old men with low testosterone and high levels of hypertension, diabetes, hyperlipidemia, and obesity. The study had to be stopped due to the high rate of cardiovascular-related events among the group on TRT (25 cases), as compared to the placebo group (5 cases)[4].This study was also highly publicized.
A second 2013 study found that 1,223 veterans who started testosterone therapy (method of administration not specified) after a coronary angiography had a 5.8% higher rate of heart attacks (myocardial infarctions) than the veterans not receiving TRT did[5]. The risk of adverse heart issues wasn’t as bad as in the first study, but concerns at the federal level were still raised.
The third study from 2014 was much broader in scope and compared the rate of non-fatal heart attacks among a sample of 55,593 men before and three months after filling a prescription for TRT at least once (method of administration unidentified). The study found that heart attacks occurred at a rate of 1.17 per 1000 patient years among men under 65 years old and at a rate of 2.19 per 1000 patient years for men over 65. The authors still concluded that TRT “substantially” increased the risk of heart attack and contributed to a twofold increase in the risk of heart attack among men 65 and older[6]. As a result, the FDA started requiring all testosterone replacement therapy product makers to note the increased risk of heart attack and stroke on the their product labels[7].
Putting Risk in Perspective
To put this “substantial” risk into perspective, I looked at the rate of patients being harmed due to preventable medical errors. A 2003 study of seniors in an ambulatory care setting found that severe harm (e.g., serious, life-threatening, or death) due to preventable medical errors occurred at a rate of 13.8 per 1000 person years[8] (6.3 times greater than the non-fatal heart attack risk associated with TRT). A 2010 study found a rate of 251 patient harm cases per 1000 admissions due to medical errors among seniors in an ambulatory care setting (114.6 times greater than the non-fatal heart attack risk associated with TRT). Finally, a 2013 study describing efforts to lessen adverse medical errors among hospitalized children reported that preventable medical errors were reduced from 33 per 1000 admissions to 15 per 1000 admissions[9] (still about 7 times greater than the heart attack risk of TRT).These three studies show that the risk of harm from receiving care inambulatory or hospital settings is far more “significant” than the adverse cardiac events associated with TRT. It makes me wonder why warning labels aren’t required on those facilities.
Even so, the risk of heart-related risk from TRT remains unclear. A 2014 study also looked at the risk of heart attacks in a population-based cohort of older men receiving TRT via intramuscular injections (not transdermal gel, orally, or other non-injectable methods). In this study, 6,355 patients treated with at least one injection were compared to a sample of non-users and followed for eight years (as opposed to a few months). The study found that testosterone therapy solely using the injectable mode of administration was not associated with an increased risk of heart attacks. In fact, a comparison of the heart attack rates among TRT users and non-users led the researcher to suggest that TRT appears to have a protective effect against heart attacks, particularly among older men at higher risk for it suffering an infarction[10].
Comparing the first three studies to this latter study suggested a possible relationship between the method of administering TRT and the risk of heart-related issues. This was clarified in a second 2014 study. This study examined and compared the risk of cardiovascular events when TRT was administered orally, via transdermal gel, and by intramuscular injection. The study found that TRT taken orally was associated with an increased risk of cardiovascular events, while TRT administered via intramuscular injection or transdermal gel was not. Thus, the risk of adverse heart-related outcomes is tied to the method of administering TRT, not the TRT itself.
Final Comments on TRT and Heart Attack Risk
TRT relative to the risk of heart attacks appears to be associated with how TRT is administered; testosterone injections or transdermal administration appear to be the safest methods.
However, I have an issue with the strong focus on risk. Let’s say that among men over 65 on TRT, two will suffer a heart attack for every 1000 using the therapy, as reported in the 2013 study above. Can’t we also say that 998 of every 1000 men aged 65 and over on TRT will not suffer from heart-related issues? It is important to look beyond the negative aspects at times, as it can potentially blow “risk” out of proportion. The truth is that despite TRT contributing in some small way to the risk of heart attack, the vast majority of men put on TRT never suffer any heart-related issues: 99.8% of older men on TRT receive the benefits with very little downside.
Prostate Cancer
Increased risk of prostate cancer has been thought of as a risk of TRT for many years, evidenced by a key 2010 study[11]. However, a second 2010 study found that out of 96 men on TRT for six years, 49 showed no increased development of the disease[12]. A 2012 study also showed the use of a transdermal TRT patch had no impact on prostate cancer risk[13]. Most recently, a detailed review of past research regarding the risk of prostate cancer and TRT identified too many study weaknesses to definitively identify an association; the researchers concluded that there is no clear evidence showing that TRT should be withheld due to an increased risk of prostate cancer[14].
Sleep Apnea
Past research has reported a connection between worsening symptoms of obstructive sleep apnea (OSA) following the start of TRT, especially in obese men[15]. As a result, TRT has generally been withheld from obese men with the condition. However, recent research examining the time course of TRT influences on OSA has shown that the adverse effect on OSA only occurs at the beginning of the therapy; it lessens dramatically after several weeks[16,17].
Part 3 of this series will address the many benefits of TRT.
[expand title=”References (click to expand)”]
- Pietrangelo, A. Bigger, Faster, Stronger? 5 Benefits of Testosterone. 2013, August. https://www.healthline.com/health-slideshow/benefits-testosterone#1
- Bassil, N., Alkaade, S., & Morley, J. E. The benefits and risks of testosterone replacement therapy: a review. Therapeutics and Clinical Risk Management. 2009; 5, 427.
- Jick, S. S., & Hagberg, K. W. The risk of adverse outcomes in association with use of testosterone products: a cohort study using the UK-based general practice research database. British Journal of Clinical Pharmacology. 2013; 75(1), 260-270.
- Basaria, S., Coviello, A. D., Travison, T. G., Storer, T. W., Farwell, W. R., Jette, A. M., … & Bhasin, S. Adverse events associated with testosterone administration. New England Journal of Medicine. 2010; 363(2), 109-122.
- Vigen, R., O’Donnell, C. I., Barón, A. E., Grunwald, G. K., Maddox, T. M., Bradley, S. M., … & Ho, P. M. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA. 2013; 310(17), 1829-1836.
- Finkle, W. D., Greenland, S., Ridgeway, G. K., Adams, J. L., Frasco, M. A., Cook, M. B., … & Hoover, R. N. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS One. 2014; 9(1), e85805.
- Brooks, M. Testosterone Labels Must Now Note CV, Stroke Risks, FDA Says. 2015, March. https://www.medscape.com/viewarticle/840811
- Gurwitz, J. H., Field, T. S., Harrold, L. R., Rothschild, J., Debellis, K., Seger, A. C., … & Bates, D. W. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003; 289(9), 1107-1116.
- Starmer, A. J., Sectish, T. C., Simon, D. W., Keohane, C., McSweeney, M. E., Chung, E. Y., … & Landrigan, C. P. Rates of medical errors and preventable adverse events among hospitalized children following implementation of a resident handoff bundle. JAMA. 2013; 310(21), 2262-2270.
- Baillargeon, J., Urban, R. J., Kuo, Y., Ottenbacher, K. J., Raji, M. A., Du, F., et al. Risk of myocardial infarction in older men receiving testosterone therapy. Annals of Pharmacotherapy. 2014; 48(9), 1138-1144.
- Pierorazio, P. M., Ferrucci, L., Kettermann, A., Longo, D. L., Metter, E. J., & Carter, H. B. Serum testosterone is associated with aggressive prostate cancer in older men: results from the Baltimore Longitudinal Study of Aging. BJU International. 2010; 105(6), 824-829.
- Leibowitz, R. L., Dorff, T. B., Tucker, S., Symanowski, J., & Vogelzang, N. J. Testosterone replacement in prostate cancer survivors with hypogonadal symptoms. BJU International. 2010; 105(10), 1397-1401.
- Raynaud, J., Gardette, J., Rollet, J., & Legros, J. Prostate-specific antigen ( PSA) concentrations in hypogonadal men during 6 years of transdermal testosterone treatment. BJU International. 2013; 111(6), 880-890.
- Gray, H., Seltzer, J., & Talbert, R. L. Recurrence of prostate cancer in patients receiving testosterone supplementation for hypogonadism. American Journal of Health-System Pharmacy. 2015; 72(7), 536-541.
- Matsumoto, A. M., Sandblom, R. E., Schoene, R. B., LEE, K. A., GIBLIN, E. C., Pierson, D. J., & Bremner, W. J. Testosterone replacement in hypogonadal men: effects on obstructive sleep apnoea, respiratory drives, and sleep. Clinical endocrinology. 1985; 22(6), 713-721.
- Hoyos, C. M., Killick, R., Yee, B. J., Grunstein, R. R., & Liu, P. Y. Effects of testosterone therapy on sleep and breathing in obese men with severe obstructive sleep apnoea: a randomized placebo-controlled trial. Clinical Endocrinology. 2012; 77(4), 599-607.
- Killick, R., Wang, D., Hoyos, C. M., Yee, B. J., Grunstein, R. R., & Liu, P. Y. The effects of testosterone on ventilatory responses in men with obstructive sleep apnea: a randomised, placebo-controlled trial. Journal of Sleep Research. 2013; 22(3), 331-336.
[/expand]